Keratoconus Treatment
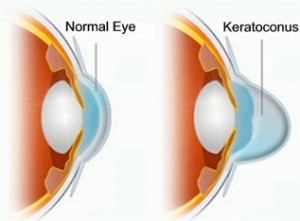
Keratoconus is an eye condition where the normally round-shaped cornea progressively thins, causing a cone-like bulge to develop. The cornea is the clear window of the eye and is responsible for focusing the light entering the eye. Therefore, distortion of the corneal shape can significantly impact everyday vision. It usually affects both eyes, but one may be more severely impacted.
It has been estimated to occur in 1 out of every 2,000 persons in the general population and can affect men and women alike. The cause of the disease is still being investigated. Genetics, allergies and other environmental factors may contribute to its onset and severity.
Keratoconus is generally first diagnosed in the late teens or early 20s, with symptoms of blurry vision with glasses, frequent glasses prescription changes, and high astigmatism. The severity of keratoconus can vary greatly, with some patients requiring only a simple glasses prescription while others can only see with special, rigid gas permeable contact lenses.
Pepose Vision doctors are corneal experts and leaders in the diagnosis and treatment of Keratoconus and the only eye center in the area that uses an advanced laser for a safer and more precise treatment and offers the absolute latest FDA-approved treatments.
How do I know if I have a keratoconus?
The earliest signs of keratoconus are usually blurred vision and frequent changes in eye glass prescription, or vision that cannot be corrected with glasses. Symptoms of keratoconus generally begin in late teenage years or early twenties, but can start at any time.
Keratoconus, especially in the early stages can be difficult to diagnose and all of the listed symptoms could be associated with other eye problems. Simply recognizing symptoms does not by itself diagnose Keratoconus, which is why we recommend a thorough examination if you have any of these symptoms and even if you don’t, every two years.
Other symptoms include:
- Increased light sensitivity
- Difficulty driving at night
- A halo around lights and ghosting (especially at night)
How do you treat keratoconus?
Intacs corneal inserts or implants are a less invasive surgical option used for correcting bulging or irregularity of the cornea, the front window of the eye. Intacs were originally approved by the FDA in 1999 to treat mild nearsightedness (myopia). However, Intacs are now primarily used to reshape bulging, thin, and/or irregular corneas. Intacs are thin, clear half-rings or semicircular segments of plastic that can be inserted into the cornea to provide support for the cornea and help make its shape more circular (instead of oval).

In 2004, the FDA granted Intacs a Humanitarian Device Exemption (HDE) to allow for surgical treatment of keratoconus. Keratoconus is a condition where the collagen in the cornea is weaker, so that the shape of the cornea changes and bulges. This approval allows Intacs to be marketed for reduction or elimination of nearsightedness and astigmatism in keratoconus patients where functional vision is no longer obtained with glasses or contact lenses.

For keratoconus patients who are contact lens intolerant, Intacs provide a new option to improve both corrected and uncorrected vision, and may defer the need for a corneal transplant. Laser vision correction, such as LASIK, is usually not an option in keratoconus because sculpting and removing tissue with the excimer laser would further thin and weaken an already weakened cornea.
Intacs procedures are performed by fellowship-trained corneal surgeons, such as the cornea specialists at Pepose Vision Institute.
Corneal Cross-Linking

Corneal Cross-Linking (CXL) is an in-office eye procedure that strengthens the cornea if it’s been weakened by keratoconus.
Alternative and brand names for the procedure include Corneal Collagen Cross-Linking, C3-R, CCL and KXL.
The minimally invasive CXL procedure involves applying liquid riboflavin (vitamin B2) to the surface of the eye, followed by treatment with a controlled application of ultraviolet light, to eliminate corneal ectasia.
Corneal Cross-Linking also can be combined with other procedures for keratoconus treatment, such as Intacs, in advanced cases.
Frequently asked questions
Why choose Pepose Vision to treat your keratoconus?
The doctors at Pepose Vision are highly experienced in the diagnosis and treatment of keratoconus, having treated thousands of patients.
Our surgeons are proud to be the FIRST and ONLY corneal specialists in the Bi-State region to utilize a bladeless technique to create Intacs channels.
Should you be diagnosed with keratoconus, we advise that the best way to ensure a successful outcome is to choose a highly experienced specialist who is involved in developing customized treatments to enhance your vision outcome.
What’s your next step?
If you think you might have keratoconus, or any vision issues, please come see us.
Our experienced doctors will use advanced diagnostics to determine exactly how to help you see your very best, patiently answer your questions and carefully explain all your options.

